Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon’s 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron
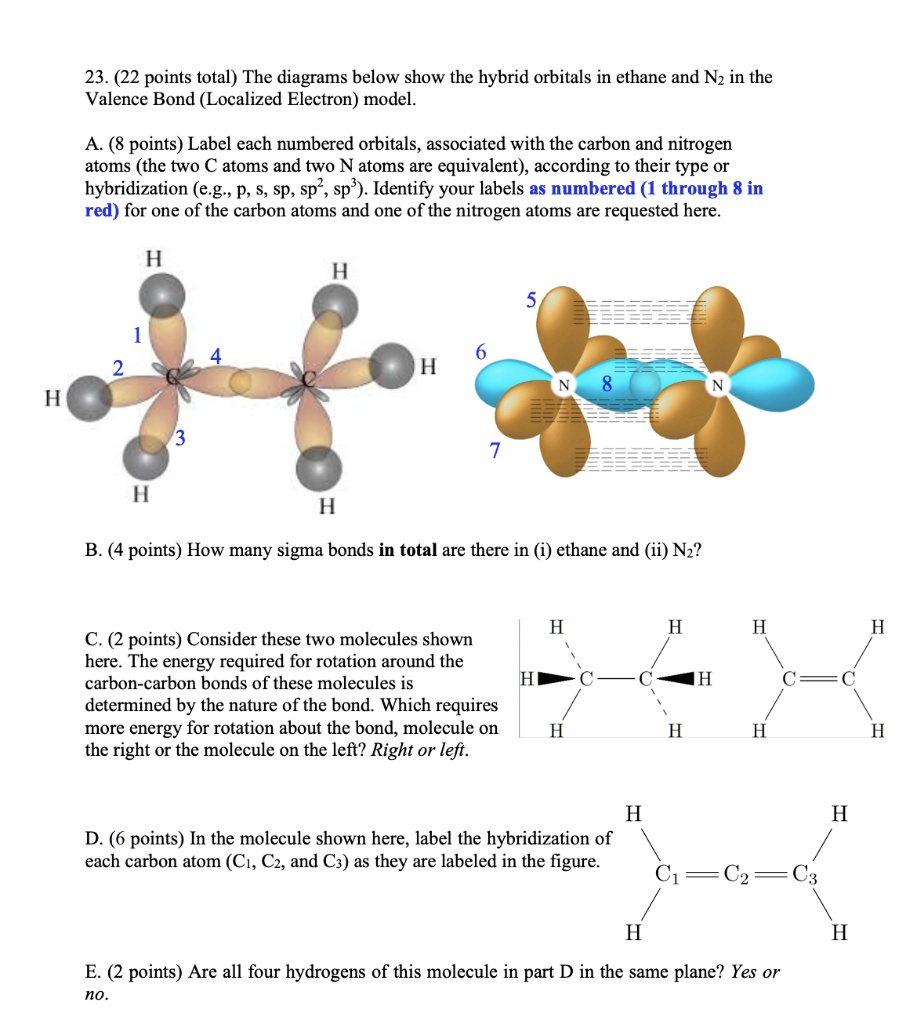
SOLVED: 23 . (22 points total) The diagrams below show the hybrid orbitals in ethane and Nz in the Valence Bond (Localized Electron) model points) Label cach numbered orbitals, associated with the
label each carbon atom with the appropriate hybridization – YouTube 0:00 / 1:54 label each carbon atom with the appropriate hybridization OneClass 13.7K subscribers

Source Image: labproinc.com
Download Image
Nov 13, 2022Trigonal hybridization in carbon: the double bond. Carbon and hydrogen can also form a compound ethylene (ethene) in which each carbon atom is linked to only three other atoms. Here, we can regard carbon as being trivalent. We can explain this trivalence by supposing that the orbital hybridization in carbon is in this case not sp 3, but is sp 2 instead; in other words, only two of the three

Source Image: selvita.com
Download Image
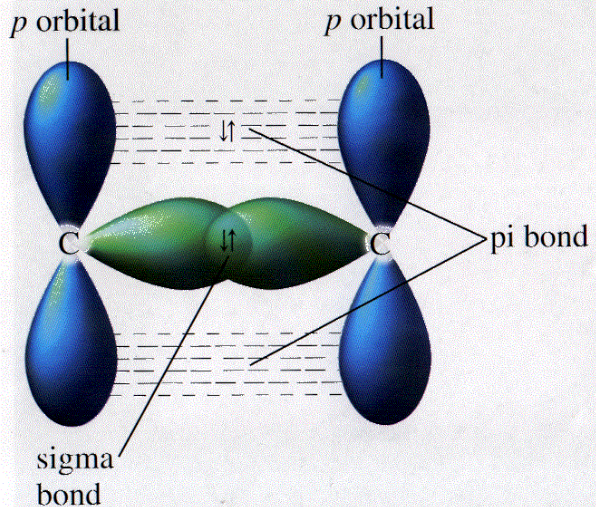
100 Nano-Stories: Sigma & Pi Bonds! | by Carlos Manuel Jarquín Sánchez | Medium What is the hybridization of the central atom in each of the following: Label each carbon atom with the appropriate hybridization. Which hybridization scheme allows the formation of at least one π bond? Identify which types of orbitals overlap to form the bonds between the atoms in a benzene molecule.
Source Image: chegg.com
Download Image
Label Each Carbon Atom With The Appropriate Hybridization.
What is the hybridization of the central atom in each of the following: Label each carbon atom with the appropriate hybridization. Which hybridization scheme allows the formation of at least one π bond? Identify which types of orbitals overlap to form the bonds between the atoms in a benzene molecule. About Transcript We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Created by Jay. Questions Tips & Thanks
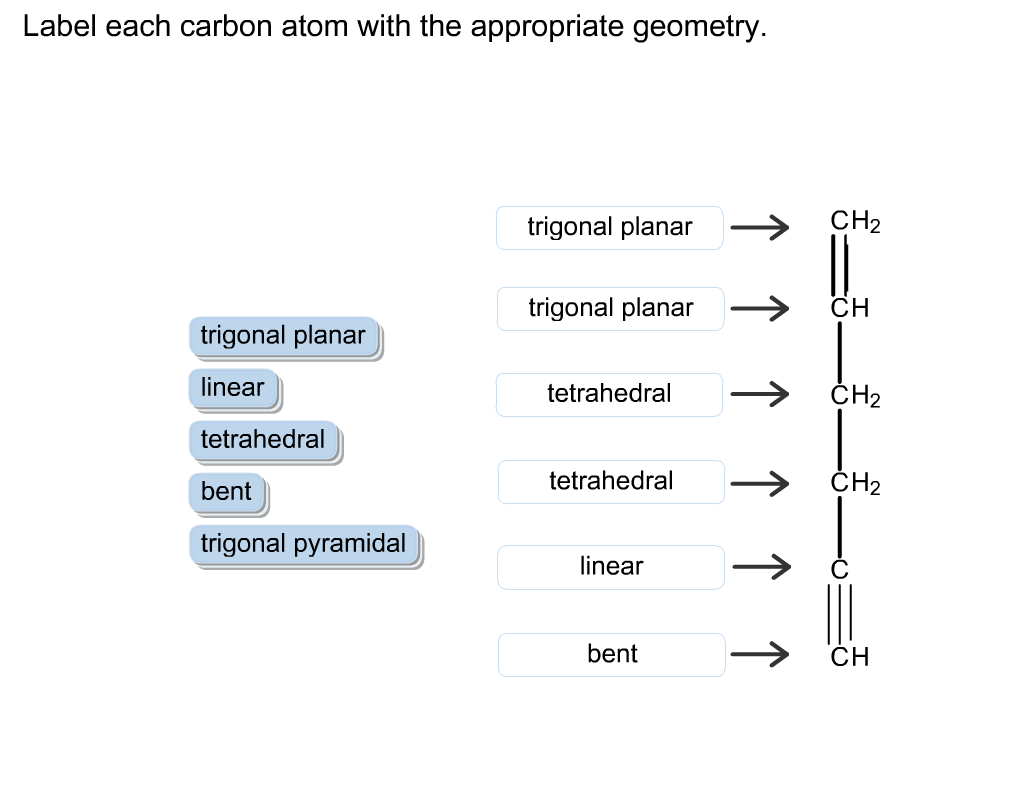
Solved Label each carbon atom with the appropriate geometry. | Chegg.com
Ball and stick model of a carbon dioxide molecule. The central carbon atom has two double bonds to oxygen atoms, and the O-C-O angle is 180 degrees. What is the hybridization around the central carbon atom in CO 2 ? Answered: What is the hybridization at each… | bartleby
Source Image: bartleby.com
Download Image
Hydroxide Molecule | Definition, Lewis Structure & Ion – Video & Lesson Transcript | Study.com Ball and stick model of a carbon dioxide molecule. The central carbon atom has two double bonds to oxygen atoms, and the O-C-O angle is 180 degrees. What is the hybridization around the central carbon atom in CO 2 ?

Source Image: study.com
Download Image
SOLVED: 23 . (22 points total) The diagrams below show the hybrid orbitals in ethane and Nz in the Valence Bond (Localized Electron) model points) Label cach numbered orbitals, associated with the Example: sp 3 Hybridization in Methane; Because carbon plays such a significant role in organic chemistry, we will be using it as an example here. Carbon’s 2s and all three of its 2p orbitals hybridize to form four sp 3 orbitals. These orbitals then bond with four hydrogen atoms through sp 3-s orbital overlap, creating methane.The resulting shape is tetrahedral, since that minimizes electron
Source Image: numerade.com
Download Image
100 Nano-Stories: Sigma & Pi Bonds! | by Carlos Manuel Jarquín Sánchez | Medium Nov 13, 2022Trigonal hybridization in carbon: the double bond. Carbon and hydrogen can also form a compound ethylene (ethene) in which each carbon atom is linked to only three other atoms. Here, we can regard carbon as being trivalent. We can explain this trivalence by supposing that the orbital hybridization in carbon is in this case not sp 3, but is sp 2 instead; in other words, only two of the three
Source Image: cjarquin.medium.com
Download Image
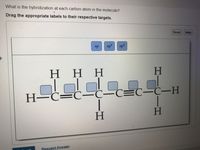
SOLVED: What is the hybridization of the indicated atom in each of the following molecules? Drag the appropriate labels to their respective targets: Reset Help sp2 sp3 sp2 0 sp CH3-CH2-CH2 CH2=CCH2 Draw a Lewis structure, predict the molecular geometry by VSEPR, and determine the hybridization of sulfur for the following: (d) H 2 SO 4 molecule (the hydrogen atoms are bonded to oxygen atoms) Solution. (a) Draw a Lewis structure. (b) Predict the geometry about the carbon atom. (c) Determine the hybridization of each type of carbon atom.

Source Image: numerade.com
Download Image
Draw The Lewis Structure For C2h2, In the lewis structure of CH 2 Cl 2 molecule, carbon atom is located as the center atom and other atoms have made bonds with carbon atom. What is the hybridization of the central atom in each of the following: Label each carbon atom with the appropriate hybridization. Which hybridization scheme allows the formation of at least one π bond? Identify which types of orbitals overlap to form the bonds between the atoms in a benzene molecule.
Source Image: atsholding.de
Download Image
Know your oligo mod: phosphorothioate bonds About Transcript We can find the hybridization of an atom in a molecule by either looking at the types of bonds surrounding the atom or by calculating its steric number. In this video, we use both of these methods to determine the hybridizations of atoms in various organic molecules. Created by Jay. Questions Tips & Thanks
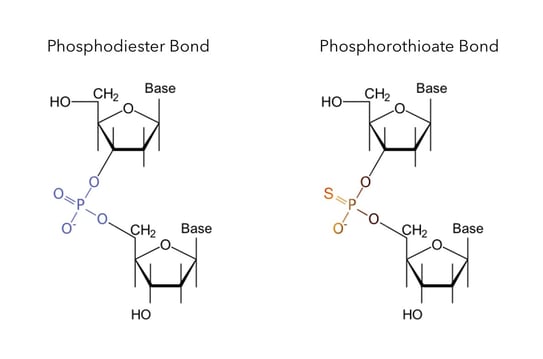
Source Image: blog.biosearchtech.com
Download Image
Hydroxide Molecule | Definition, Lewis Structure & Ion – Video & Lesson Transcript | Study.com
Know your oligo mod: phosphorothioate bonds label each carbon atom with the appropriate hybridization – YouTube 0:00 / 1:54 label each carbon atom with the appropriate hybridization OneClass 13.7K subscribers
100 Nano-Stories: Sigma & Pi Bonds! | by Carlos Manuel Jarquín Sánchez | Medium Draw The Lewis Structure For C2h2, In the lewis structure of CH 2 Cl 2 molecule, carbon atom is located as the center atom and other atoms have made bonds with carbon atom. Draw a Lewis structure, predict the molecular geometry by VSEPR, and determine the hybridization of sulfur for the following: (d) H 2 SO 4 molecule (the hydrogen atoms are bonded to oxygen atoms) Solution. (a) Draw a Lewis structure. (b) Predict the geometry about the carbon atom. (c) Determine the hybridization of each type of carbon atom.