to rank the given compounds in order of increasing value Bility, we need to simply solve for the Moeller Celje bility. In the case of barium carbonate, we get one mole of berry Mayan one mole of carbonate ion For every mullah barium carbonate that dissolves Therefore Caspi is going to be equal to the product of barium concentration multiplied bicarbonate concentration.
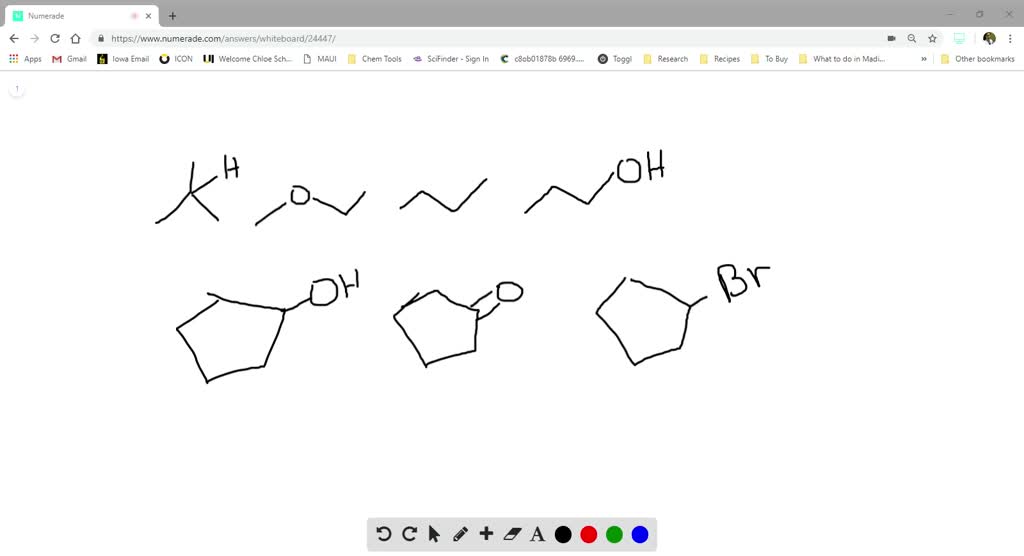
SOLVED: Rank the following compounds in order of increasing water solubility. Their Lewis structures are given below. Make sure to make the order of your ranking clear: Isopropanol (CH3CHOHCH3) H OH |
Therefore, the order of increasing water solubility is: Br < 2 < OH Br is the least soluble because it cannot form hydrogen bonds with water. 2 is slightly more soluble than Br because it has more electrons, which means it can form slightly stronger London dispersion forces with water. … Rank the following compounds in order of increasing
Source Image: numerade.com
Download Image
Rank the following organic compounds in order of increasing solubility in water. #1 being the MOST soluble and #4 being the LEAST soluble. Give an explanation for each of your ranking choices. Be sure to include both concepts of intermolecular forces and polarity in your response. propanoic acid propane propanal 1-bromopropane

Source Image: atlas-scientific.com
Download Image
Arrange the compounds in the order of increasing solubility in water (least soluble first) A) III,IV,II,I B) II,III,IV,I C)IV,III,II,I D)III,IV,I,II | Homework.Study.com
Rank the following compounds in order of increasing water solubility. Solution Summary: The author explains that the solubility of a given organic compound in water is determined by how many polar functional groups are present in it or its hydrogen bonding capability with water. The author explains that the solubility of a given organic compound in

Source Image: pubs.acs.org
Download Image
Rank The Following Compounds In Order Of Increasing Water Solubility
Rank the following compounds in order of increasing water solubility. Solution Summary: The author explains that the solubility of a given organic compound in water is determined by how many polar functional groups are present in it or its hydrogen bonding capability with water. The author explains that the solubility of a given organic compound in
Chemistry Chemistry questions and answers Rank the following compounds in order of increasing solubility in water? Select 4 (most soluble) for the most soluble compound and 1 (least soluble) for the least soluble compound in water. Compounds Increasing solubility in water Х 3 Methanol (CH3OH) 4 (most soluble) 12 3 MgBr2 1 (least soluble) F. 2 2
Screening Ionic Solvents for Enhancing the Solubility of Water-Insoluble Natural Dyes | Industrial & Engineering Chemistry Research
21. Nuclear Chemistry 2h 32m. 22. Organic Chemistry 5h 6m. 23. Chemistry of the Nonmetals 2h 39m. 24. Transition Metals and Coordination Compounds 3h 14m. Arrange the following compounds in order of their expected increasing solubility in water: Br2, KBr, toluene (C7H8, a constituent of gasoline).
Answered: Rank the following compounds in order… | bartleby

Source Image: bartleby.com
Download Image
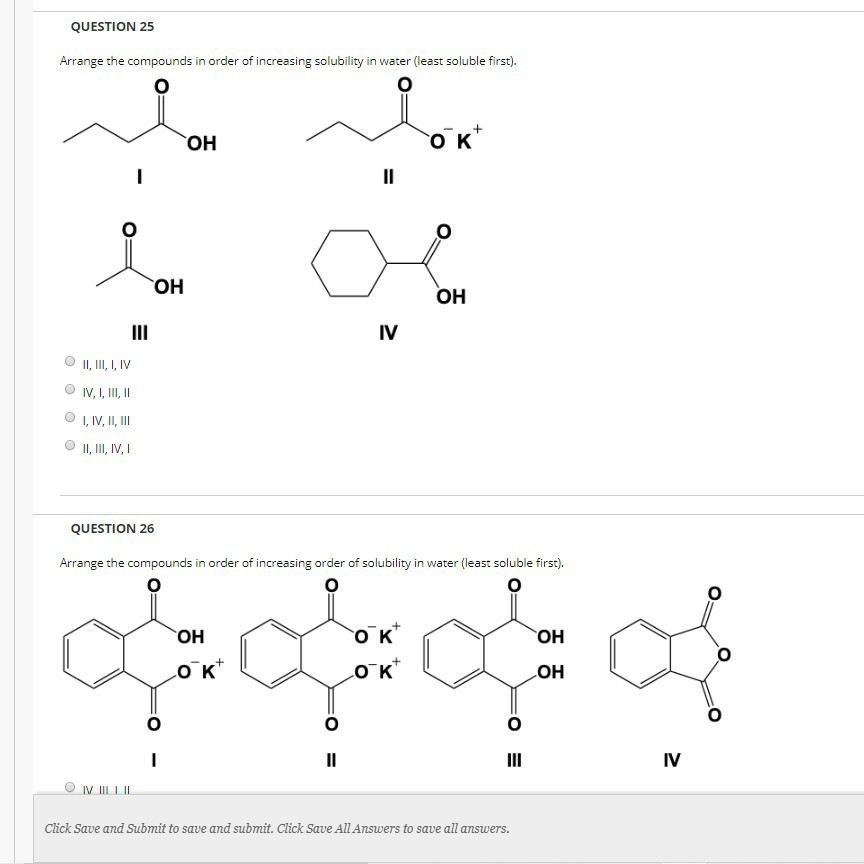
Solved QUESTION 25 Arrange the compounds in order of | Chegg.com
21. Nuclear Chemistry 2h 32m. 22. Organic Chemistry 5h 6m. 23. Chemistry of the Nonmetals 2h 39m. 24. Transition Metals and Coordination Compounds 3h 14m. Arrange the following compounds in order of their expected increasing solubility in water: Br2, KBr, toluene (C7H8, a constituent of gasoline).
Source Image: chegg.com
Download Image
SOLVED: Rank the following compounds in order of increasing water solubility. Their Lewis structures are given below. Make sure to make the order of your ranking clear: Isopropanol (CH3CHOHCH3) H OH |
to rank the given compounds in order of increasing value Bility, we need to simply solve for the Moeller Celje bility. In the case of barium carbonate, we get one mole of berry Mayan one mole of carbonate ion For every mullah barium carbonate that dissolves Therefore Caspi is going to be equal to the product of barium concentration multiplied bicarbonate concentration.

Source Image: numerade.com
Download Image
Arrange the compounds in the order of increasing solubility in water (least soluble first) A) III,IV,II,I B) II,III,IV,I C)IV,III,II,I D)III,IV,I,II | Homework.Study.com
Rank the following organic compounds in order of increasing solubility in water. #1 being the MOST soluble and #4 being the LEAST soluble. Give an explanation for each of your ranking choices. Be sure to include both concepts of intermolecular forces and polarity in your response. propanoic acid propane propanal 1-bromopropane

Source Image: homework.study.com
Download Image
⏩SOLVED:Rank the water solubility of the following compounds. | Numerade
However, their water solubility is still limited. Step 3/3 3. Ionic compounds: Ionic compounds are highly water-soluble because they dissociate into ions in water, which can interact with water molecules through ion-dipole interactions. Therefore, the order of increasing water solubility is: Nonpolar compounds < Polar compounds < Ionic compounds.
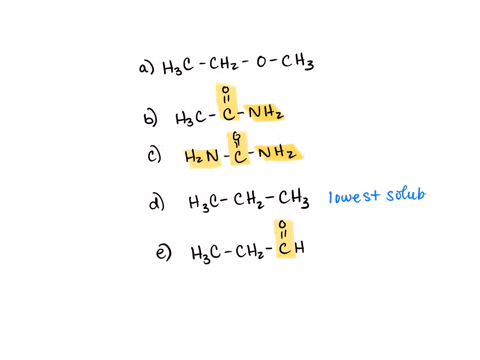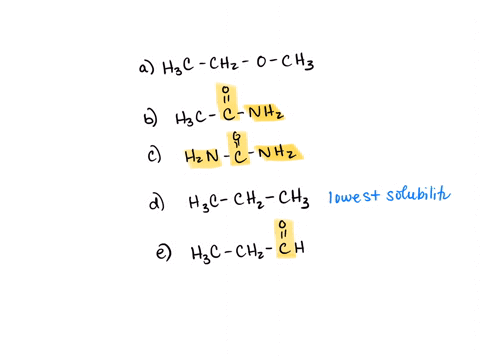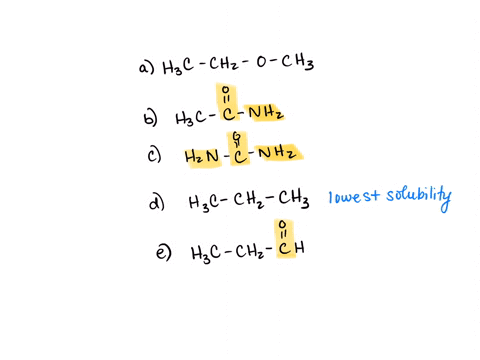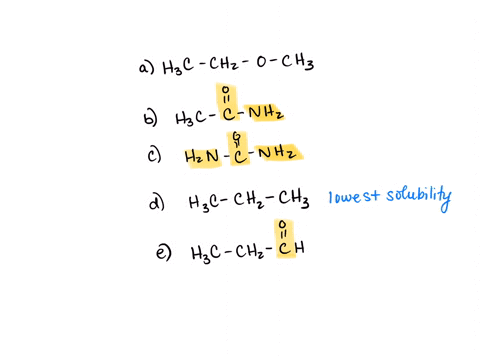
Source Image: numerade.com
Download Image
Answered: Rank the following compounds in order… | bartleby
Rank the following compounds in order of increasing water solubility. Solution Summary: The author explains that the solubility of a given organic compound in water is determined by how many polar functional groups are present in it or its hydrogen bonding capability with water. The author explains that the solubility of a given organic compound in
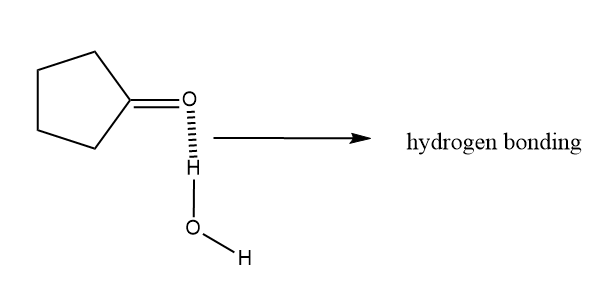
Source Image: bartleby.com
Download Image
Arrange the following compounds in order of their expected increa… | Channels for Pearson+
Chemistry Chemistry questions and answers Rank the following compounds in order of increasing solubility in water? Select 4 (most soluble) for the most soluble compound and 1 (least soluble) for the least soluble compound in water. Compounds Increasing solubility in water Х 3 Methanol (CH3OH) 4 (most soluble) 12 3 MgBr2 1 (least soluble) F. 2 2

Source Image: pearson.com
Download Image
Solved QUESTION 25 Arrange the compounds in order of | Chegg.com
Arrange the following compounds in order of their expected increa… | Channels for Pearson+
Therefore, the order of increasing water solubility is: Br < 2 < OH Br is the least soluble because it cannot form hydrogen bonds with water. 2 is slightly more soluble than Br because it has more electrons, which means it can form slightly stronger London dispersion forces with water. … Rank the following compounds in order of increasing
Arrange the compounds in the order of increasing solubility in water (least soluble first) A) III,IV,II,I B) II,III,IV,I C)IV,III,II,I D)III,IV,I,II | Homework.Study.com Answered: Rank the following compounds in order… | bartleby
However, their water solubility is still limited. Step 3/3 3. Ionic compounds: Ionic compounds are highly water-soluble because they dissociate into ions in water, which can interact with water molecules through ion-dipole interactions. Therefore, the order of increasing water solubility is: Nonpolar compounds < Polar compounds < Ionic compounds.